Nonferrous Alloys in Shipbuilding

Aluminum Alloy Applications
Aluminum alloys find use where their special attributes such as low density and high strength to weight ratio, corrosion resistance in certain environments or retention of toughness at low temperatures are of value. Development of inert gas arc welding processes has facilitated the use of aluminum alloys for various ship structural applications. Aluminum alloys are frequently used in superstructures of large ships and for the entire hull structure of some ferries and small boats such as those serving the offshore industry.
The low density of aluminum alloys makes them particularly attractive for applications where high strength to weight ratios are of particular concern such as in surface effect and hydrofoil craft. Since aluminum alloys increase in strength and maintain toughness as temperature decreases, they have proven particularly suitable for cryogenic services such as containment of liquefied natural gas. Details of compositions, properties and methods of inspection are contained in American National Standards Institute documents...
Non-Heat Treatable Aluminum Alloys
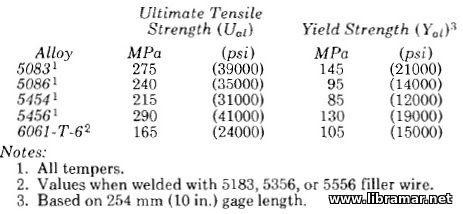
Aluminum-magnesium alloys which are the alloys most widely used for marine structures, 5083 (4.5% Mg), 5086 (4.0% Mg), and 5456 (5.0% Mg) alloys, acquire increased strength from cold work and not from heat treatment. The 5454 alloy is used for applications where service temperatures above 65°C (150°F) are anticipated. These alloys, which have good weldabilily characteristics, are usually used in the mildly cold worked (1/4 hard) temper to provide the desirable combination of strength and corrosion resistance.
Higher strength forms of these alloys, attained either by additional cold work or by magnesium contents over 5 percent are not generally used, since they tend to exhibit an undesirable increased susceptibility to stress corrosion. Where special corrosion problems are anticipated, such as in stagnant bilge areas, the alloys may be provided in special tempers particularly resistant to exfoliation, a special form of intergranular corrosion which produces delamination.
In general, the base plate in the vicinity of welds in non-heat treatable alloys, such as the 5XXX series, are transformed to an annealed condition by the heat of welding. The effect is to reduce tensile properties in the vicinity of the weld to the annealed or non-work hardened values. The effect, which should be taken into account in design, is reflected by the fact that the minimum ultimate tensile strength properties expected for transverse butt welds are annealed plate properties.
Heat Treatable Aluminum Alloys
Heat treatable aluminum alloys such as 6061-Т6 develop strength by heating to an annealing temperature, water quenching and then reheating to a lower temperature to achieve a controlled precipitation of intermetallic compounds. The 6061-T6 alloy is occasionally used in marine service, particularly for extrusions, since it extrudes more readily than the 5083 or 5086 non-heat treatable alloys.
The strength of the 6061-T6 alloy is higher than that of the 5083 or 5086 alloys; however, in the 6061-T6 alloy, the strength, ductility and corrosion resistance of the area in the vicinity of welds are severely degraded by the heat of welding. The extent of degradation is indicated by the reduction from the specified minimum tensile strength of 289 MPa (42,000 psi) for 6061-T6 base plate to the 165 MPa (24,000 psi) indicated for the weld joint in Table. Such adverse effects limit the applicability of the 6061 alloy for welded applications.
Corrosion of Aluminum Alloys
Aluminum alloys generally do not experience excessive corrosion under normal operating conditions. However, aluminum alloys in contact with dissimilar metals, may corrode at an accelerated rate. Such conditions may occur between faying surfaces of aluminum and other metals, between aluminum hulls and non-aluminum piping or when non-aluminum piping passes through aluminum bulkheads, decks, etc. In such cases, aluminum should be isolated from the other metal by means of suitable non-water absorbing insulating tapes or coatings or gaskets or by use of special pipe hangers or fittings. The 1974 SOLAS Convention contains certain stipulations on the use of aluminum.
Aluminum in contact will, wood, insulating materials or concrete should be protected against the corrosive effects of impurities in these materials by suitable coverings or coatings; concrete should he free of additives for cold weather pouring. Suitable precautions should be taken to avoid arrangements that could induce crevice corrosion in wet spaces. In certain stagnant water applications, such as bilge spaces or chain lockers where exfoliation corrosion may not be of concern, use of the alloys specially heat treated to resist this form of corrosion should be considered. For generalized protection, sacrificial anodes or cathodic protection systems may be considered. To minimize adverse corrosive effects of stray currents, it is advisable, when possible, to keep such systems in operation while the vessel is in dock.
Fire Protection
Compared with steel, aluminum alloys have relatively low melting points and tend to lose strength rapidly upon exposure to elevated temperatures. In considering use of aluminum, due consideration should be given to applications where retention of structural integrity would be required in fire exposure. The use of appropriate insulation protection should be considered for such applications.
Copper-Nickel Alloys
Copper-nickel alloys have been used as solid plate to 10 mm (0.375 in.) thick, and as copper-nickel clad steel in thicknesses over 10 mm (0.375 in.) for small boats; copper-nickel clad steels have been suggested for large seagoing vessels. Significant economic advantages of copper-nickel hulls were reported for a fleet of small, 20-m (65-ft) shrimp boats operating in highly fouling Central American waters. Benefits were attributed to eliminating frequent drydocking for removal of barnacles and repainting, and to avoidance of reduction in speed and operating efficiency associated with increased hull frictional resistance derived from barnacle growth and accumulation. Steel hulls have been reported to be inferior to copper-nickel hulls in the following respects:
• Barnacles grow and accumulate on steel,
• steel eventually corrodes in service,
• cleaning and repainting of a steel hull does not restore the hull to its original smoothness; this results in increasing hull resistance.
The retention of hull smoothness over the life of a ship, which may he provided by a copper-nickel hull, could result in cost benefits associated with increased operating speeds and decreased fuel consumption. An economic study has indicated that the potential annual cost benefit of a copper-nickel hull, compared with a steel hull, may possibly justify the extra cost of the copper-nickel material for some larger ships with high capital costs such as liquefied natural gas carriers and roll-on/roll-off ships.
To date the alloy used has been 90% copper- 10% nickel; new alloys with lower copper and higher nickel contents which offer promise of increased resistance to cavitation erosion have been proposed as possible candidates for the larger ships. For larger ships, where plate thickness exceeds 6.3 mm (0.25 in.), it is generally more economical to use copper-nickel clad steel instead of copper-nickel plate.
The "Read Later" function allows you to add material to this block with just one click. Just click on the icon and read the articles that interest you at any convenient time.